Specifications of copper metal
Copper is a chemical element with the symbol cu and has a very high flexibility and thermal conductivity. Copper is used as a building material and as a building material for various metal alloys such as sterling silver.
Copper has the chemical properties of transition metals, has oxidation states of +1 and +4, its compounds are colored, with atomic number 29 and atomic mass of 546/63.
Various physical properties including copper grade, amount of natural impurities or residues of additives formed at the boundary of solid solution grains or separate phases; Mechanical and thermal pretreatment of metal, which leads to conditions such as cast copper, hot tubular copper and hard copper, molding copper, spitting copper, are other features of copper metal.
This element is one of the few metals that can be used in nature as a metallic form. For this reason, it has been used by humans in different regions throughout history. Of course, this is not only because of its abundant resources in nature but also because of its excellent properties.
The metal is currently used in electricity, electronics, energy, petrochemicals, transportation, machinery, metallurgy and other new industries and some advanced fields.
Copper metal producing countries
List of copper producing countries |
|||||||||
| Rank | Country | Rank | Country | Rank | Country | Rank | Country | Rank | Country |
| 1 | Chile | 2 | United States | 25 | India | 26 | Turkey | 49 | Albania |
| 3 | Peru | 4 | China | 27 | Botswana | 28 | Burma | ||
| 5 | Australia | 6 | Indonesia | 29 | Pakistan | 30 | Armenia |
|
|
| 7 | Russia | 8 | Canada | 31 | Philippines | 32 | Georgia | ||
| 9 | Zambia | 10 | Poland | 33 | Finland | 34 | Romania | ||
| 11 | Kazakhstan | 12 | Iran | 35 | North Korea | 36 | Vietnam | ||
| 13 | New Guinea | 14 | Argentina | 37 | Serbia | 38 | Spain | ||
| 15 | Brazil | 16 | Congo | 39 | Macedonia | 40 | Namibia | ||
| 17 | Mongolia | 18 | Mexico | 41 | Mauritania | 42 | Morocco | ||
| 19 | Uzbekistan | 20 | Bulgaria | 43 | Tanzania | 44 | Zimbabwe | ||
| 21 | South Africa | 22 | Sweden | 45 | Japan | 46 | Cyprus | ||
| 23 | Portugal | 24 | Laos | 47 | Saudi Arabia | 48 | Columbia | ||
History of copper in the world and Iran
Copper is the first element discovered by man after gold, and the copper tools discovered date back to around 5000 BC. There are indications that copper was melted and purified from its oxides, such as malachite and azurite, by 5000 BC.
Copper was first discovered in ancient Cyprus. Copper was mined in Cyprus and has been used in various industries since that time. Before the advent of metal smelting technology and knowledge, people heated copper metal and pounded it into sheets.
The sheets were then sharpened with a hammer, resulting in the first copper knives. And so began the history of copper. The oldest metal objects found in present-day Iraq are in the area between the Tigris and Euphrates, which was a copper pendant and is estimated to be 8700 years old.
Properties of copper metal
Copper, silver and gold are in group 11 of the periodic table. These three metals have high flexibility and electrical-thermal conductivity. The metal is usually made in the form of fine-grained polycrystalline, which is stronger than single-crystal forms.
The softness of this metal partly explains its high electrical and thermal conductivity. After silver at room temperature has the highest temperature conductivity. This is due to the low resistance of its electrons to mobility after heating.
Copper is one of the few metallic elements with a natural non-gray or silver color. Pure copper is reddish-orange in color and produces reddish spots when exposed to air. The color of this metal is due to the electronic transfer between the filled atomic shells d3 and 4S semi-empty.
Because the energy difference between these shells corresponds to the orange light. Like other metals, galvanic corrosion will occur if this element comes in contact with another metal.
The metal does not react with water but reacts slowly with atmospheric oxygen to form a layer of brownish-black copper oxide that protects the underlying metal from further corrosion, unlike iron rust.
The green layer (copper carbonate) that is often seen in old structures such as the roofs of many old buildings and the Statue of Liberty is copper oxide. The metal reacts with certain sulfur compounds when they are exposed to form various sulfides.
Copper alloys
Many alloys have been formulated for this metal, many of which have important applications. For example, brass is an alloy of copper and zinc. Bronze is commonly referred to as copper-tin alloys, but can also refer to any other alloy, such as aluminum bronze.
This metal is one of the most important solder compounds of gold and silver that is used in the jewelry industry and changes the color, hardness and melting point of the resulting alloys. Some lead-free solders are made of tin alloyed with small amounts of copper and other metals.
Copper and nickel alloys in low value coins are often used for exterior cladding. The alloy of 90% copper and 10% nickel is very significant due to its corrosion resistance and is used for various objects that are exposed to seawater.
However, it is vulnerable to pollutant sulfides that are sometimes found in ports. In addition, copper alloys with aluminum (about 7%) are golden in color and are used in decoration.
The following table lists the important alloys of this metal:
| Alloy name | Combined elements | Uses |
| brass | Zinc | Screws, wires, pipes, electrical fittings, musical instruments and decorative accessories |
| Bronze | Tin | Sculptures, electrical connections, springs and pins |
| Phosphor Bronze | Tin and phosphorus | Bearings, springs, percussion instruments, instrument wires |
| Bronze aluminum | Tin, aluminum, iron, nickel and bronze | Tools, engine parts for cars and airplanes |
| Copernicus | Nickel, iron, manganese | Coins and utensils |
| Warsaw ( Nickel Silver ) | Zinc and Nickel | Keys, coins, wind instruments – brass |
Various applications of copper metal
The main applications of copper are power generation (60%), roofing and plumbing (20%) and industrial machinery (15%). Copper is mostly used as a pure metal , but when more hardness is needed, it is placed in alloys such as brass and bronze.
The metal has been used in boats and hulls for more than two centuries to control the growth of plants and oysters. A small portion of copper resources are used for food supplements and fungicides in agriculture. This metal is also used in machinery, but it is mostly used as an alloy, which is described below.
-
Application in the electrical industry
One of the most widespread applications of this metal is in power transmission devices such as wires and cables, transformers, switches, components and two-way connections, etc.
In addition, this material is used in the construction of motors, for example as stators, rotors, as well as hollow wires, communication cables and residential electrical circuits.
-
Application in the electronics industry
Vacuum electronics such as high-frequency and high-frequency tubes, cross-catheters, magnetrons, etc. use this metal. Copper printed circuits require a large amount of copper foil, and in integrated circuits this metal has been replaced by aluminum in silicon chips for joints.
-
Application in energy and petrochemical industries
The main condenser pipes and plates in coal-fired power plants are made of brass, bronze and copper-nickel. Solar heaters are also often made of copper tubing. Various types of containers for storing corrosive intermediates, piping systems, filters, pumps and valves, types of evaporators and condensers and heat exchangers in the petrochemical industry are made of this metal alloy.
Due to its resistance to corrosion and in the form of water-soluble copper ions, this substance has antiseptic properties and can protect marine organisms from pollution. This element and its alloys are widely used in desalination systems and offshore drilling and other underground installations.
-
Application in the transportation industry
Alloys such as aluminum bronze, manganese bronze, aluminum , brass, tin, zinc and nickel copper (monolith) alloys are standard materials in shipbuilding. Gears, bearings, brake pads, electrical and power systems, washers and all kinds of fittings and accessories, etc.
in trains, motors, rectifiers and controls, brakes, electrical and signal systems for copper and its alloys They rely on.
In addition, rail power is a major source of demand for copper and its alloys. Wiring, hydraulic pneumatics and aircraft cooling systems all require this metal.
-
Application in mechanized and metallurgical industries
Movable and fixed parts such as cylindrical liners, gears and fittings require the use of copper or its alloy for flexibility and lubrication. Also, an important part of metallurgical equipment in continuous casting technology-crystallizer is made of chromium copper and silver copper or other alloys of this metal, which have high strength and conductivity.
Also, by adding a small amount of this metal to structural steel, the hardness of the steel and its corrosion resistance in air and water can be improved. Adding this material to cast iron and stainless steel means more resistance to corrosion.
-
Application in the health system
This biostatic metal is bactericidal and many other forms will not grow alive on it. For this reason, it has long been used for parts of ships to protect against algae and oysters.
Alloys of this metal are used as an important material in the production of mesh in the aquaculture industry. Because they are antimicrobial and structurally resistant to corrosion even in harsh conditions.
Copper production stages
The production of this metal comes from three main stages :
- Concentration of ore
- Conversion of sulfides of this metal and other compounds to copper
- Copper purification
-
Concentration of ore
Ore enrichment is done using the “Flotation method“. The powdered rock is mixed with an organic solvent such as oil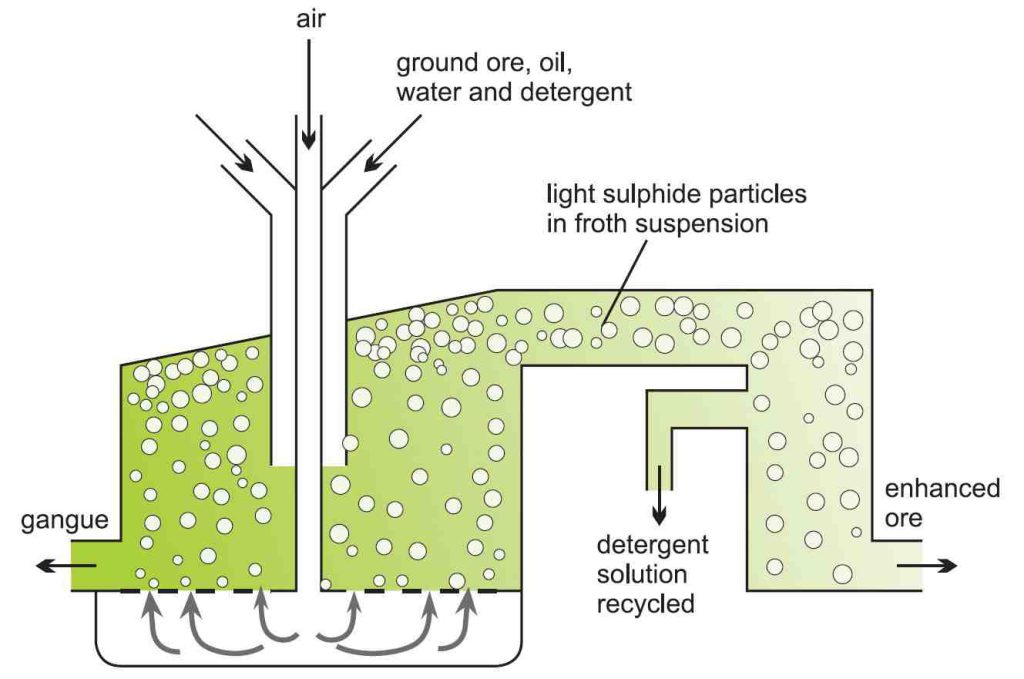 and then mixed with water and detergents in large tanks.
and then mixed with water and detergents in large tanks.
Compressed air is introduced into the mixture, and the light sulfide components of the metal rise and float on the floor.
Heavier clays and other silicates that settle to the bottom of the tank are called “Gangue“. Eventually, the copper-rich foam will come out of the tanker.
-
Conversion of copper sulfides and other compounds
This conversion is done through several methods as follows:
- Burning (roasting) of copper sulfide ore
- Leaching process
- Bacterial method
A) Burning (roasting) of copper sulfide ore
The enriched ore is burned with enough air to convert iron sulfide to iron (II) oxide. The solid mixture is mixed with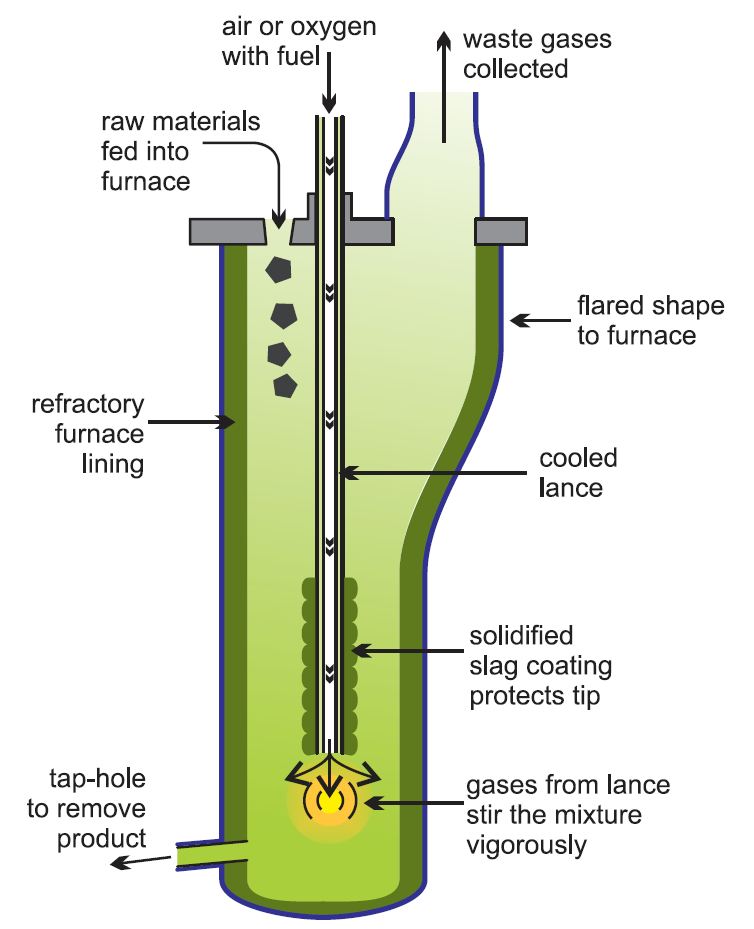 calcium carbonate and sand (silica) and heated to 1026° C.
calcium carbonate and sand (silica) and heated to 1026° C.
Iron forms silicate slag, and copper (I) sulfide is melted and placed at the bottom of the furnace. This molten material is called “Copper Matte“.
In a newer method called “ISASMELT“, condensed rock, lime and silica, together with a solid fuel such as coal, form compounds and pellets.
The pellets are sent into the furnace, where a tube pumps natural gas (methane) along with oil and oxygen-rich air into the furnace.
Economically, it is better to use pure oxygen or oxygen-rich air instead of air, as this will speed up the reactions and, consequently, use smaller plants with lower fuel costs.
In addition, using pure oxygen will make it easier to control contaminants such as sulfur dioxide.
The resulting mixture is pumped rapidly to the bottom, causing a turbulent flow and a high reaction rate. This process is highly efficient and large quantities of raw materials can be processed this way with relatively small furnaces.
Matte copper and slag are transferred to another kiln for settling and separation. Then, a matte copper is introduced into another furnace and air or oxygen-rich air is blown into it to produce the metal.
Typically, sulfur dioxide is converted to sulfuric acid on site. Copper GDP, ” blister copper” say. The material is heated to melt and sulfur will be removed by injecting extra air. This is accompanied by the injection of methane to remove oxygen. If crude copper is still present, it is purified by electro-refining.
B) Leaching process
By washing the ore with a solution of copper (II) chloride and iron (III) chloride, copper (I) chloride is obtained :
Sodium chloride is added to the compound to retain copper (I) chloride in solution. In the presence of excess chloride ions, the CuCl2 complex will be formed, which is soluble in water. Finally, crude copper is obtained by electrolysis of CuCl2 solution and copper (II) chloride is recycled.
C) Bacterial method
Significant amounts of this metal produced in the United States are obtained using bacteria. Acidic water is sprayed on copper mineral waste. These wastes contain small amounts of copper. As water passes through the crushed rocks, bacteria break down iron sulfides into iron (II) and iron (III) ions.
After that, iron (III) ion oxidizes copper sulfide ion to sulfate and copper (II) ion remains in solution. Copper-rich water is recycled and metallic copper is obtained by a reduction process.
-
Purification of
Whatever method is used to extract this metal, the final purification process is done by electrolysis. Impure copper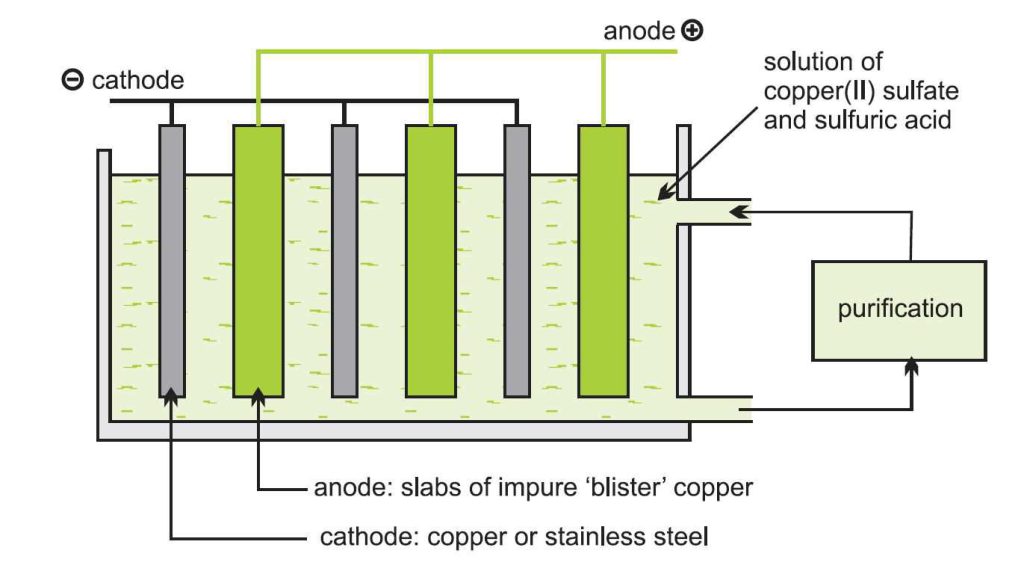 plates with sheets of this pure metal, stainless steel or titanium, are immersed in a solution of copper (II) sulfate and sulfuric acid.
plates with sheets of this pure metal, stainless steel or titanium, are immersed in a solution of copper (II) sulfate and sulfuric acid.
Pure copper or steel sheets, the cathode of the electrolysis cell, and impure plates form the anode of the cell. This means that ions of this metal are formed at the anode and enter the solution.
The ions that migrate to the cathode are reduced to pure copper and deposited at the cathode. Over time, pure metal is removed from the cathode.
Many impurities in the anode, such as gold, silver, platinum, and tin, are insoluble in the electrolyte solution and therefore will not deposit on the cathode and settle to the bottom of the tank.
These materials are separated from the tank and sent to processing units. Other metals, such as iron and nickel, are soluble, and as a result, the electrolyte solution must be continuously purified to prevent the deposition of unwanted metals on the cathode.
With this method, we will have a purity of 99.99%. Finally, pure metal is converted into ingots for factory use.
Secondary production
This metal and its alloys, which contain large amounts of copper, are recycled for greater purity. Metals are melted by heating with oxygen-rich air. In this way, most metals except copper and precious metals are oxidized to form slag that can be removed.
The Isasmelt method described above is commonly used for the secondary production of this metal. Finally, the obtained copper, which has a purity of about 99%, is sent to the anode to be purified using the Essamlet electrolytic method.
About 33% of the copper produced in the world is obtained from the recycling of this metal. 31% of this amount is supplied by the United States and 47% by Western Europe. Half of this amount comes from factory waste and the other half from old waste such as copper pipes and wires.
If you need more information or want to buy copper from Iran please contact us. we are copper wholesaler in Iran and we can give best copper price.
