What is the meaning of stainless steel?
In the science of metallurgy, stainless steel is called stainless and steel, also known as a large family of steels that contain at least 11% chromium have. In fact, this element is the most important alloying element and a kind of versatile in these steels.
The presence of this element prevents the reaction between iron and oxygen, which results in corrosion, rust and degradation of steel.
In addition to chrome, in various grades of stainless steel; Elements such as carbon, molybdenum, nitrogen, aluminum, silicon, phosphorus, sulfur, copper, nickel, selenium and niobium are also present.
These elements are added or removed from the steel composition during casting according to the conditions and according to the needs of the industries.
Corrosion resistance of stainless steel is due to the formation of a passive thin film (passive film) that is formed on the surface in the presence of chromium. The presence of this inactive and sticky layer makes the steel immune to corrosion attacks and even repairs itself in the event of damage or scratches.
In addition, with some changes in the chemical composition of this alloy, this resistance can be exceeded, including:
- Increase the percentage of chromium to a greater extent (more than 11%)
- Addition of nickel to the chemical composition in the amount of more than 8%
- Add molybdenum to the chemical composition
Stainless steel has good rust resistance, low maintenance costs and a polished and shiny surface. In addition, due to the high strength of these steels, many large and small industries need to consume and compete for quality production between advanced industrial countries such as Japan; Germany and India have risen.
Because their production requires special knowledge and technology. Today, these steels are produced and marketed in various forms such as sheets, plates, rods, pipes, etc.
Familiarity with different types of stainless steels
-
Stainless steel ferrite
Ferritic stainless steels are steels with 10.5 to 27% chromium and less than 0.5% nickel. The microstructure of these steels consists of a ferrite (α) phase with a crystalline structure (BCC).
Due to the high percentage of chromium, the crystal structure usually remains the same at different temperatures. The structure of BCC has the property of becoming a magnet and can absorb magnets. That is why these steels are magnetic.
These steels do not have the ability to strengthen using heat treatment methods. Also, strengthening them using hard work method will not have much effect on increasing their strength.
Chromium is an element that plays a key role in increasing the corrosion resistance of these steels. This element, in addition to the presence of molybdenum, forms a compound resistant to creep, stress corrosion and corrosion against acids.
For this reason, ferritic steels are used in refineries and petrochemicals as pipelines for the transportation of petroleum products. In addition, they are used in the manufacture of car exhausts, agricultural implements and home appliances.
-
Austenitic stainless steel
Austenitic stainless steels are known for their nickel, manganese and chromium alloying elements. Usually these steels have about 16-20% chromium and 7-20% nickel.
Nickel is a phase stabilizer. For this reason, these steels are composed of austenite phase with FCC structure at all temperatures. Of course, the presence of carbon and nitrogen greatly helps to increase the stability of the austenite phase.
The presence of nickel has created many advantages for austenitic steels. The presence of this alloy maintains the strength of the alloy at high temperatures and greatly increases the corrosion resistance of the alloy.
For this reason, these alloys are used in the manufacture of heat furnaces, pipelines for the transfer of chemicals such as hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3) or heat exchangers.
These steels are not capable of hardening by heat treatment methods. Because the nickel phase is stable and unchanging at all temperatures. Also, these steels do not have magnetic properties.
-
Martensitic stainless steel
These steels are carbon and chromium based alloys and about 12-17% chromium is found in their structure. These steels are produced by the rapid cooling of austenitic steels and are therefore known as martensitic stainless steels.
Martensitic steels have the highest percentage of carbon among stainless steel alloys. The presence of this amount of carbon causes the formation of chromium carbide in the grain boundaries of steel and the formation of this phase leads to increased corrosion.
For this reason, martensitic stainless steel, unlike ferrite and austenitic types, does not have high resistance to corrosion. In contrast, strength, toughness, toughness and high fatigue strength are the strengths of these steels.
For this reason, these steels are used in the manufacture of spoons, knives, springs, tool steels and abrasion resistant steels.
At a glance, these steels can be classified into 4 categories:
- Chromium-plated steels: These steels were among the first martensitic steels to be produced and are still the most widely used among martensitic stainless steels. Chromium is the main alloying element in the structure of this category. These steels are used in common engineering applications and in cases where high creep resistance is required.
- Chromium-nickel steels: Similar to the previous category, except that some of the carbon has been replaced by nickel in the structure. This increases the toughness and corrosion resistance of these alloys. Samples with high nickel content are used in turbines.
- Hardening scale steels: These steels have the ability to harden and martensitize together. The strength and fracture toughness of these alloys are good and the aerospace industry is one of their flagship applications.
- Creep resistant steels: As the name implies, these steels have high creep resistance and are used in high temperature applications where creep intensifies. This is achieved by adding a small amount of boron, cobalt, vanadium and niobium to the main composition.
Heat treatment in martensitic stainless steel
Martensitic steels have a brittle structure after production. For this reason, after production, one or more stages of heat treatment should be performed on them to maintain their hardness and improve their other mechanical properties.
This operation is performed in three stages:
- Austenitizing
- Quenching
- Tempering
In this method, the alloy is generally heated to a temperature range of 980-1050° C to form an austenitic structure with an FCC structure. After forming this structure, the steel is cooled in air or oil to find a martensitic structure with a BCT crystal structure.
Finally, the steel temper will be done at 315 degrees Celsius to find the necessary flexibility. However, if more flexibility is required, this can also be done in the temperature range of 590-620° C. In this case, as the toughness and flexibility increase, the hardness of the steel decreases.
The important point in the heat treatment of these steels is that this should not be done between the temperature range of 370-590 degrees. Because by changing the structure of chromium carbide (Cr7C3), the impact strength and corrosion resistance of these alloys are reduced.
-
Duplex stainless steels
These steels are known as two-phase because their structure is a combination of two phases, ferrite and austenite. These alloys are known for their high levels of chromium and molybdenum. The amount of chromium in these alloys is between 19-32% and molybdenum is maximum 5%.
These steels have a set of properties of good corrosion resistance, high strength and reasonable price. According to experiments, the yield strength of these steels is almost twice that of austenitic steels. The stress corrosion resistance of these steels is excellent due to the presence of ferrite phase in their structure.
Stress corrosion (SCC) under conditions such as the presence of chlorides, humidity or high temperatures can cause problems for steel. Therefore, 304 and 316 austenitic steels perform better in these conditions.
Also, due to the reduction of nickel, their production costs are cheaper. Today, the oil and gas industries are the main customers of this category of steel.
-
Stainless steels are hardening deposits
Hardening scale steels have been developed due to limitations in increasing the strength of austenitic and ferritic stainless steels and increasing the toughness of martensitic steels.
Based on the microstructure, hardening scale steels can be divided into 3 categories: martensitic hardness steels, semi-austenitic hardness steels and austenitic hardness steels.
-
Stainless steel hardens martensitic sediment
Sediment steels have a very high hardness and are commonly used in the form of wires, rebars, sheets and heavy parts in the forging process. These steels have a martensitic structure at room temperature.
However, ferrite is sometimes observed in the composition of these alloys, which can be eliminated by homogenization and austenitization of the steel.
After converting the structure to martensite, the hardness can be increased again during the hardening deposition operation by depositing a secondary phase in the structure.
-
Stainless steel semi-austenitic hardening scale
These steels are called semi-austenitic because they have an austenitic structure in the annealed state, but are converted to martensite with a suitable heat treatment.
The martensitic structure can be formed by rapid quenching of the part or severe cold operation of the part. Then the aging operation at high temperature (for example 510 ° C) causes the deposition of the secondary phase and increases the hardness of the alloy.
-
Stainless steels austenitic hardening sediment
Austenitic steels have an austenitic structure during solidification and this structure remains stable at all temperatures. This is due to the high presence of nickel in the structure, which stabilizes the austenite phase. The increase in strength in these steels is done by dissolution and aging operations.
Note that the aging time is longer than martensitic and semi-austenitic hardening deposition steels. Because the penetration of alloying elements in the field of austenite is slower than martensitic and semi-austenitic steels.
8 steps to produce stainless steel
-
Melting of raw materials
All raw materials, including steel scrap and ferrous alloys including ferrochrome, ferronickel, ferromanganese,  ferromolybdenum and ferrosilicon, are mixed and melted in an electric arc furnace (EAF).
ferromolybdenum and ferrosilicon, are mixed and melted in an electric arc furnace (EAF).
By separating the slag and sediment, the melt is refined and ready for the next step.
-
Decarbonization of the melt
Depending on the grade that must be present in the steel composition, the carbon in it is adjusted and the rest is removed from the structure as carbon monoxide in reaction with oxygen gas.
The required oxygen is supplied by blowing in the gas.
The reaction for removing carbon from the melt is as follows:
4Cr(bath) + 3O2 → 2Cr2O3(slag)
Cr2O3(slag) + 3C(bath) → 3CO(gas) + 2Cr(bath)
-
Continuous Casting ( CC )
The refined melt is continuously cast in a mold and after solidification is cut into slabs, billets or blooms.
-
Forming
In the next step, the produced ingot is taken to the shaping workshops to become the final product. The operation begins with the hot rolling of steel products. First, the slab is heated and then it is passed through large rollers.
The ingots and ingots turn into rods and wires after forming, but the slabs are usually turned into products such as plates, sheets and coils.
-
Heat treatment
After casting and forming operations, problems such as residual stresses and high density of defects occur in the part, which increase its energy and make it malleable. For this reason, annealing heat treatment is performed on the parts to release residual stresses and reduce energy.
In addition, some grades of stainless steel require special operation to achieve good mechanical properties. An example of this operation can be seen for stainless steels of martensitic hardening deposition, which increases its strength with hard deposition operation.
The execution of this operation depends on the type of steel structure that we examined in the section and is different for each category.
-
Removal of surface oxides
Despite the many advantages of the annealing process, during this process, due to the reaction that occurs between the surface of the part and oxygen; The surface of the part is oxidized, which in addition to adverse effects on mechanical properties, reduces the beauty of the surface of the part.
For this reason, these oxides must be removed from the surface. This can be done mechanically by abrasion of the part surface or by using acidic and alkaline solutions.
-
Cutting
After the removal of surface oxides, large slabs are cut into smaller dimensions to be ready for use in manufacturing industries. Cutting can be done mechanically using cutting machines such as guillotines or using heat, plasma and etc.
-
All work
In the last stage, the surface polishing operation is performed on the steel so that the surface smoothness of the part is higher and it has a more beautiful appearance.
Usages of stainless steel
Looking around, we can see the use of these steels in the construction of the simplest tools needed by humans, such as spoons, to build the body of very complex and sensitive parts such as spacecraft.
In this section, we refer to the usages of this alloy in some areas that are more significant for us today.
-
3D printing of metals
3D printing is a method based on making materials layer by layer and connecting them through the freezing process. Stainless steels are one of the alloys that benefit from this method.
An obvious example is 316 alloy, which is printed by this method due to its high freezing rate and very fast heat transfer.
-
Military equipments
Stainless steel is used in the manufacture of weapons of war due to its high resistance to corrosion and oxidation. Some guns are made entirely of stainless steel, such as the M1911pistol.
These alloys have low wear, scaling and oxidation rates and are therefore a good choice for making weapons.
-
Chemical industries and refineries
Industries such as oil, gas and petrochemicals that deal with corrosive chemicals cause the most corrosion and damage to parts. In some units, such as distillation towers, where the temperature sometimes rises to 800 degrees Celsius, corrosion becomes more severe.
Therefore, the use of simple carbon steels does not meet these needs and stainless steel should be used.
In addition, industries such as factories dealing with acid production, plastics, fertilizers, and… dealing with chemicals must use these steels in their facilities.
-
Architecture and construction
The use of stainless steel in the construction industry is quite obvious to all of us. Handles, railings, elevators and even in the exterior facades of buildings, these steels are used for high strength, beauty and excellent corrosion resistance.
With the development of these steels, especially the austenitic and duplex types, we should see a wider presence of their products in our homes.
-
Usage in the aerospace industry
One of the challenges that manufacturing engineers always face is the use of alloys with a high strength to weight ratio that can meet the maximum expectations in working conditions.
Resistance to corrosion, fatigue, creep and oxidation are some of the things that alloys used in missiles, airplanes and spacecraft must be able to withstand. By meeting these needs, Steel has been able to be used in the construction of engines, fuselages, skeletons and nozzles of aircraft and rocket fuel tanks.
-
Containers for food and beverages
It must have occurred to you to eat on steel plates. These containers are usually made of 304 or 316 stainless steel alloys. However, sometimes ferritic or martensitic stainless steels (400 series) are also used for this purpose.
The advantage of using these steels is that they do not affect the taste of food and drink and are easy to clean and disinfect. Therefore, they do not pose a problem for human health.
-
Manufacture of agricultural implements
The use of fertilizers, pesticides and pesticides creates corrosive conditions on farms. In this case, if the equipment used is not corrosion resistant, it will rust and be damaged after a short time.
The use of stainless steel, especially austenitic type, for the manufacture of machinery and chemical storage containers is an effective step in reducing corrosion.
-
Medical Equipment
The field of medicine and dentistry is one of the fields in which stainless steel has played a very colorful role. Many surgical instruments, such as blades and needles, are made of steel today. These parts are highly durable and can be easily sterilized.
In addition, these steels are widely used in the manufacture of surgical implants, roots, crowns and orthodontic wires, because in these conditions, biological corrosion reactions and mechanical wear cause rapid erosion of the part and the part used must be Last for years.
-
Energy field
Stainless steels are widely used in the construction of equipment for all power plants. These materials are an ideal choice when there is liquid or gas penetration in the equipment.
Examples of these materials can be found in water cooling filters, hot gas purifiers, and electrolysis devices that produce hydrogen from water molecules by electroplating.
Martensite is commonly used when equipment requires high strength, but austenitic and ferritic steels are a good choice for applications with less stress and more corrosion resistance.
-
Decorative tools
The use of stainless steel to make decorative items such as jewelry, watches and decorative objects is very common. Grade 316L is one of the most popular types used for this purpose.
Impact of alloying elements on the properties of stainless steels
It is very important to know the alloying elements in stainless steels, because the properties of these steels, especially the mechanical and chemical properties of the steel, are directly affected by its alloying elements. It is sometimes observed that the addition of an element up to a few tenths of a percent of the properties of steel upside down.
For this reason, metallurgical engineers should be familiar with the naming system of steels and also the effect of each of these elements when ordering so that they do not face any problems after preparing the steel.
In general, chromium, nickel and carbon are among the main elements that are added to the steel composition. In addition, elements such as molybdenum, silicon, vanadium, aluminum, niobium, tungsten, nitrogen, manganese, copper and cobalt are other elements found in the composition of steels. Each of these elements has its own special effects, which we will examine in this section.
-
Chromium (Cr)
As we mentioned in the introduction to this article, by adding more than 10.5% chromium, the steel is in a stainless state. When this value reaches above 12%, chromium prevents the steel from rusting by creating an oxide layer on the steel surface.
By replacing the BCC and FCC crystal lattices, this element increases the strength of the solid solution, increases the wear resistance by forming a hard phase of chromium carbide, and slightly increases the toughness of the steel.
The presence of this element in high amounts, impoverishes the structure of chromium and reduces its corrosion resistance, which we will discuss in the relevant section.
-
Nickel (Ni)
Nickel is an austenitic element. Therefore, we should mostly see the presence of this metal in austenitic stainless steels. However, this element can also be seen in other steels.
This element simultaneously increases the hardness, hardness, tensile strength and toughness of steel. In addition, the presence of this element by more than 5% increases the corrosion resistance and oxidation of steel. Finally, increasing the soft-to-brittle transition temperature (DBTT) is another benefit of this element.
-
Manganese (Mn)
Manganese is an austenitic element. In combination with sulfur, this element forms manganese sulfide (MnS) and reduces its adverse effects such as brittleness and hot brittleness after casting the alloy. Increased stiffness, stiffness and tensile strength are other benefits of this element.
-
Molybdenum (Mo)
Molybdenum is a ferritic element. Molybdenum in ferritic, austenitic and biphasic steels in amounts of more than 6% increases the corrosion resistance, especially the pitting corrosion in sulfur and chloride environments. This element is carbide and its effect can be well seen in martensitic steels.
These carbides increase the strength of martensite and increase the corrosion and creep resistance of austenitic and duplex stainless steels.
-
Silicon (Si)
Silicon, like molybdenum, stabilizes the ferrite phase. During casting, this element is used as an oxygenator. In addition, it helps to improve the fluidity of the melt during casting. Improves corrosion and oxidation resistance if added at 2 to 5% to the steel composition.
This element, in combination with iron and chromium, forms intermetallic compounds such as Cr3Si that make the structure brittle.
-
Nitrogen (N)
Nitrogen is an austenitic element. This element not only significantly increases the yield strength of steel, but also increases corrosion resistance, especially pitting corrosion.
-
Copper (Cu)
Copper is an austenite phase stabilizing element. Alloys that contain the element copper are usually subjected to rigid heat treatment to achieve full strength. In addition, hard deposition in these alloys increases the corrosion resistance of the alloy in acidic and chloride environments such as seawater.
-
Titanium (Ti)
Titanium is a carbide stabilizer in stainless steel, but prevents the formation of austenite. These carbides are highly stable and prevent the formation of intergranular corrosion.
-
Phosphorus (P)
It is sometimes observed that phosphorus is added along with a small amount of sulfur to improve the tensile strength and machinability of steel. But too much of this element can be problematic. Including the effect of increasing corrosion of metals and their failure during welding.
-
Sulfur (S)
Sulfur also has a similar effect to phosphorus on the properties of steel. Therefore it must be used very carefully in the alloy composition.
-
Niobium (Nb)
Niobium, like titanium, is a carbide stabilizer in this type of steel. In addition, the addition of this element increases the resistance of these alloys to creep at high temperatures.
-
Selenium (Se)
The role of this element is to improve the machining capability of these steels. Tungsten, tantalum and vanadium also increase the strength at high temperatures by forming fine and dispersed carbides. These elements are the stabilizers of the ferrite phase.
How is stainless steel named?
Naming of steels in different standards is done in different ways. In addition to international standards that have been developed for global use, some countries have developed their own standards for naming.
Therefore, in addition to training in international standards, training in national naming standards is also essential for engineers in any country.
Naming based on ASTM system
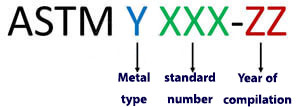
In this system, the naming of steel is done with a combination of numbers and letters, each of which introduces the characteristics of the alloy.
In this system, the letter A is used to name the base iron alloys, instead of the Y symbol. The three digits X represent the standard number, which is an ordinal number and has nothing to do with the properties of the alloy. Finally, the last two letters of the Z indicate the year of review or publication of the standard.
Naming based on AISI/SAE system
A 3-digit number is used to name stainless steel according to this standard. The first digit indicates the steel group and the next digits indicate the subdivisions of that group.

When naming according to the AISI system, in addition to this 3-digit number, suffixes are seen next to the steel name.
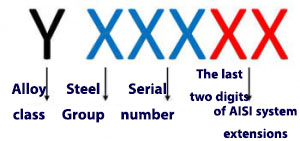
Naming based on UNS system
According to this system, steel naming is done using a letter and a five-digit number. If the steel is stainless, the first letter is denoted by S. After this letter;
The first 3 digits of the designation on stainless steels are similar to what was done in the AISI naming system for these steels. The last two digits are equivalent to the AISI system extensions mentioned in the table in the previous section.
Naming based on DIN system
This system is defined by the German Standards Committee. This naming system, like the UNS system for carbon, low-alloy and high-alloy steels, is done in different ways. To name stainless steel with this system, the following method should be used:
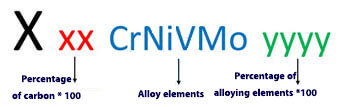
For example : X 10 CrNi 188 steel is steel with 0.1% Carbon, 18% chromium and 8% nickel, or X 20 CrN 13 steel is steel with 0.2% carbon and 13% chromium.
Status of stainless steel production in Iran and the world
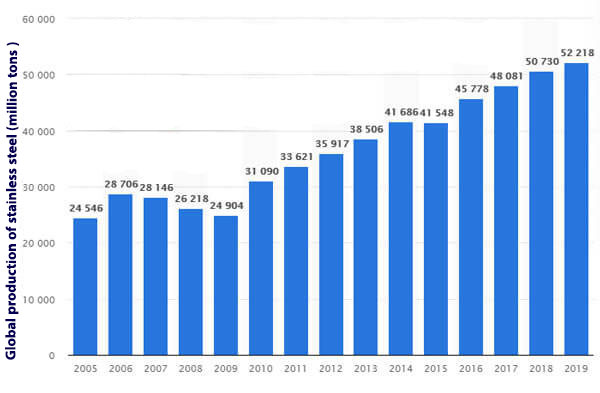
Stainless steel is a product that is expected to increase annually due to the growth of industrial development in industrialized countries. Statistics show that the production of stainless steel in 2019 was about 2.52 million tons.
Whereas in the last decade, the production of this steel has always been less than 30 million tons. The chart below shows the growth trend of these steels over the past years.
In Iran, however, the situation is different. Despite this, the production of carbon steels has had a significant trend in recent years, but the production of this type of steel has not been seriously pursued and the country’s needs are often met by countries such as India and China.
One of the problems in this field is the supply of nickel required for the production of these steels, which must be supplied from abroad.
However, Iran Alloy Steel Company has been producing this category of steels since 1997. It is hoped that with the development of production technology of this steel in the country’s industry and making the necessary investments, in the coming years we will see self-sufficiency in supplying this type of steel for our country’s industry.
Conclusion
Stainless steel has exceptional properties for special construction applications, which is a combination of inherent durability with beauty, strength, malleability and formability.
Its high price is a justification for ongoing research to maximize the properties of this metal. More work needs to be done to develop guidelines for construction applications and to be able to use more economical grades of stainless steel such as thin duplex (low nickel) and ferrite grades.
The move towards more sustainable development has opened new doors for the stainless steel industry, from the construction of power plants to buildings with high thermal efficiencies.
Research and development activities should advance these ideas and prove that stainless steel has a unique and long-term role in meeting human needs while maintaining the quality of the natural environment.
