What is Zamak
Zamak is a type of zinc-based alloy commonly used in pressurized parts. Approximately 95% of the alloy is composed of zinc, 4% of aluminum and 1% of copper. However, in the chemical analysis of this alloy, small amounts of iron, 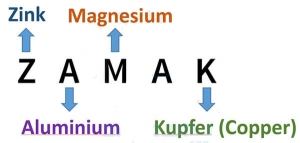 cadmium, tin, nickel, chromium and manganese can also be observed.
cadmium, tin, nickel, chromium and manganese can also be observed.
Zamak alloys are a member of the zinc-aluminum alloy family. This alloy was first introduced to the field of metallurgical science in 1929 by the Zinc Company of New Jersey.
Zamak has different grades, of which Zamak 2, Zamak 3, Zamak 5 and Zamak 7 have the most industrial use . Zamak is an acronym for the main elements of this alloy in German.
Characteristics of Zamak alloy
The element has a white color with a blue or silver sheen. The same color, appearance and some properties of zinc can be seen to some extent in Zamak alloy. This alloy is a suitable alloy for casting due to its low melting point and good fluidity. Zamak melting temperature is about 450 degrees Celsius and its production is possible in electric and flame arc furnaces.
The mechanical properties of Zamak alloy are very good and the hardness of this alloy is higher than some alloys. Also, the toughness of this alloy is really impressive. So that the specimens under tensile test show high strength from high. Zamak can withstand oxidation at temperatures up to 200° C.
How to produce Zamak alloy
To produce Zamak alloy, first an aluminum melt is prepared and then pieces of pure copper are thrown into the melt. It is necessary to raise the temperature so that all the copper dissolves inside the aluminum.
Once the AL-cu mixture is ready, it is milled. This mixture is brittle. The properties of aluminum in combination with zinc make this alloy one of the most widely used aluminum and zinc alloys and has many applications.
Once the ingots are completely frozen, melt the zinc in another plant. The frozen Al-cu Amijan ingot is then thrown into the molten bush. It is necessary for the ingot to go down and dissolve completely inside the melt. Once the alloy melt is ready, it is cast.
Due to the fact that Zamak alloy has a very short freezing range, it has a high tendency to crust. This makes compression and die casting more popular for the production of this alloy. Feeding and freezing are usually easy to determine.
The specific gravity of this alloy is high. Therefore, it is necessary not to forget about weightlifting in compression systems. Zamak alloy is a suitable alloy for casting due to its low melting point, good fluidity and concentrated shrinkage.
Mechanical properties of different grades ZAMAK
| Zamak 2 | Zamak 3 | Zamak 5 | Zamak 7 | |
| Ultimate tensile strength (Mpa) | 397 | 268 | 331 | 285 |
| Surrender strength (Mpa) | 361 | 208 | 295 | 285 |
| Percentage increase | %6 | %6.3 | %3.6 | %14 |
| Shear strength (Mpa) | 317 | 214 | 262 | 214 |
| Hardness (Brinell) | 130 | 97 | 91 | 80 |
| Modulus of elasticity (GPa) | 96 | 96 | 96 | 96 |
| Density (Kg/dm3) | 6.8 | 6.7 | 6.7 | 6.7 |
Zamak alloy elements (according to ASTM B 240 standard)
| Zamak 2 | Zamak 3 |
Zamak 5 |
Zamak 7 |
|
| Aluminium | 4.3 – 3.9 |
4.3 – 3.9 | 4.3 – 3.9 | 4.3 – 3.9 |
| Copper | 2.9 – 2.6 | 0.1 – 0 | 1.25 – 0.75 | 0.1 – 0 |
| Magnesium | 0.05 – 0.025 | 0.05 – 0.025 | 0.06 – 0.03 | 0.02 – 0.01 |
| Lead | 0.004 – 0 | 0.004 – 0 | 0.004 – 0 | 0.002 – 0 |
| Cadmium | 0.003 – 0 | 0.003 – 0 | 0.003 – 0 | 0.002 – 0 |
| Tin | 0.002 – 0 | 0.002 – 0 | 0.002 – 0 | 0.001 – 0 |
| Iron | 0.075 – 0 | 0.035 – 0 | 0.075 – 0 | 0.075 – 0 |
The effect of alloying elements on the properties of the alloy
-
Zinc
On the main element is Zamak alloy. The higher the amount of this element in the alloy, the higher the strength of the alloy. Adding this element also increases the corrosion resistance of the alloy.
Why Zinc?
- Low price of zinc mold
- Low zinc prices in international markets
- Low melting temperature resulting in low cost and easy casting
- Production of parts with high dimensional accuracy
- Casting of very thin walls up to 0.5 mm thick
- High speed of zinc casting (due to the ability to cast under hot pressure)
- Ease of plating and coating with zinc
- Long life of zinc molds (life of zinc molds used is 10 times longer than aluminum molds)
- Reduce the pores of the final parts
- Fine-grained final structure
-
Aluminum
After zinc, aluminum plays a major role in Zamak. This element increases the tensile strength, creep resistance and impact strength of the alloy. In addition, the addition of this element increases the hardness of the alloy. Note, however, that increasing this element by more than 5% will greatly increase the brittleness of the alloy.
Aluminum increases the fluidity of the alloy. So that if the amount of this element is less than 3.5%, the molten fluidity of the alloy is reduced and it must be cast at higher temperatures.
In any case, the amount of Zamak aluminum must be carefully controlled. Because if its value is more than 3.5 to 4.3 percent, it will negatively affect the mechanical properties of the alloy.
-
Copper
Increased tensile strength, creep, hardness and corrosion resistance are the results of adding copper to Zamak. In general, copper alloys have lower impact strength.
Therefore, we try to limit the amount of copper in zinc alloys under pressure (except Zamak 2) to 1.25 percent. Note that the increase in copper in this alloy also forms the brittle phase of Al2 Cu and in addition to reducing flexibility, will also reduce the dimensional accuracy of the cast alloy.
-
Magnesium
Magnesium increases the hardness of the alloy. But it reduces the impact strength, elongation percentage and fluidity of the alloy melt. It has been seen in some cases that this element is also used for deoxygenation. Magnesium increases the corrosion resistance of the alloy.
But even with a small amount of nickel, this goal can be achieved. However, the nickel element in the alloy forms the Ni2Al3 compound , which will increase the hardness and brittleness of the final part by increasing the amount of molten slag.
-
Iron
Iron is generally recognized as an impurity in pressurized castings. Because the solubility of iron in zinc-aluminum alloy is low. Excess amounts of iron increase slag at the top of the melt (for example, the FeAl3 compound accumulates at the top of the melt).
The iron element also increases the brittleness of cast alloys and creates problems in machining the final part.
-
Cadmium & Tin
These elements are known as insidious agents in the alloy composition. Because they do not show their destructive effects at the very beginning. The effects of the compounds of these elements usually appear over time and cause problems such as cracking and tearing in the part, especially in humid weather.
-
Nickel & Chromium & Manganese
These elements in the melt form the compounds Mn2Al5 , CrAl4 , Ni2Al3 , which are the main compounds of molten slag. These elements in combination with aluminum increase the slag. According to research, these intermetallic compounds, in most cases, reduce the solubility limit.
Is Zamak the same as dry lead?
In the Iranian market, Zamak is also known as dry lead, but this word is wrong. Dry lead is an alloy of lead and antimony that has nothing to do with Zamak.
Interestingly, due to some properties of lead and iron, they are the most dangerous elements in the composition of Zamak.
Because the melting point of lead is lower than Zamak and it does not dissolve at all on zinc. In case of incomplete dissolution, it goes to the grain boundaries and accumulates in those areas, causing hot failure of the part.
In what cases is Zamak used?
As mentioned, Zamak alloy is often used in parts that are pressed. Parts such as door and window components or car handles. To better understand the use of this alloy, consider the piece “Espanolite”:
Espanolites are a type of fixture that is usually used in double-glazed windows, with several small tabs along the length of the profile, to lock the door or window frame to the frame. In this way, by engaging the Zamak, the door or window sash adheres more firmly to its frame.
Zamak also plays an important role in regulating double-glazed doors and windows. The made parts must be adjusted properly so that the adjustment of the double-glazed door and window is not a problem. In addition, Zamak in double-glazed doors and windows can be repaired and replaced, and there are no restrictions.
In addition to these types of applications, due to the beautiful appearance of this alloy, it is also used in the manufacture of decorative parts such as toys and some jewelry and rhinestones. This alloy is also used in mechanical, electrical and automotive industries as corrosion-resistant (galvanized) coatings.
Reasons for using Zamak plating
-
Beautiful work
One of the main applications of plating is in beautifying the surface of metals. Due to the softness of precious metals such as gold, it can not be used in the strength parts of structures. For luxury and beauty work, gold plating is used on these parts. This process is also widely used in making and decorating watches for beautification.
An example of beautification by plating is plating in car body design. Although the use of chrome in the car body is due to the use of anti-wear properties of this material, but today, chrome coating has a visual appeal among car fans, and therefore customer’s request for this coating on their car is for beauty. Other measures taken in the automotive industry to design luxury and custom cars are plating the car body with gold.
-
Prevent chemical corrosion
One of the most important reasons for using plating is to prevent chemical corrosion of materials. Hence, this is done in two ways. One is that the parent metal is coated with a metal that has a high resistance to corrosion, and the other method is coated with a metal that has more corrosion properties than the parent metal.
In the first method: The use of metals with low corrosion properties as a coating is like coating an iron lover with silver.
In the second method: The most common example of which is white iron ( galvanized iron ), the steel is coated with a layer of zinc that is more corrosive than steel to be corroded instead of steel in the face of corrosion.
-
To increase surface resistance
Metals that have low surface resistance, both in terms of impact and abrasion resistance, are protected by a hard metal coating, such as chrome in the wear protection of the car body cover.
Another example of hard anodizing on lightweight aluminum structures in the aerospace and missile industries can be mentioned to increase the surface strength of this material.
-
Increase thermal resistance
Not only metal materials are used in plating. Today, working in high-temperature environments has made it virtually impossible to use metals to make tools. Therefore, the high resistance of ceramics to heat has made this view come to mind to use ceramic coating on metal tools and parts. Therefore, ceramic plating on metals is very important.
-
Increase electrical conductivity
Today, the world of technology and information processing speed has increased that in the design of electrical components can not be used even copper, which has a high conductivity.
In fact, to improve system processing and reduce resistive heat in processors, materials with higher conductivity and lower strength should be used, so in this industry today, gold, silver and platinum plating on steel parts are used to increase electrical conductivity.
When buying an Zamak alloy, pay attention to the following points
The price of Zamak ingots and other Zamak parts varies based on the internal fluctuations of the dollar. For this reason, you need to get the price of this product by contacting the contact numbers on the website of the companies in question.
As mentioned, Zamak alloy is available in different grades in the market. Buyers of this alloy, according to their required quantities, should refer to the table of alloy elements and the table of mechanical properties to purchase their desired grade.
If you need zinc ingot with best price in Iran please contact us.
