Zinc Metal Specification
Zinc Metal the shiny metal element is bluish white. At normal temperatures, it is brittle and crystalline, but at temperatures between 150-110 ° C, it has malleable properties. In terms of abundance, it is on the twenty-third element in the Earth’s crust.
Zinc is a bluish-white metal that darkens due to humidity and produces a glossy green color during combustion. Zinc is the fourth most widely used metal in the world after iron, aluminum and copper, etc.
Its main sources are sulfide, zinc boland or zincite sphalerite, calamine (zinc silicate) and smith sonite (zinc carbonate). Sulfide rock is roasted to be oxidized and heated with coal to 1200 ° C. It evaporates and condenses outside the reaction chamber and is molded into molds called splitters.
Zinc extraction method is obtained by burning and oxidizing zinc ore and the reaction of zinc oxide with coal or carbon by metal distillation. Zinc has 5 stable isotopes in nature and 16 unstable isotopes are known for zinc.
This element has a semiconductor property and burns very hot in the air with a red flame and emits toxic white clouds. The formability of this element is high. Neither zinc nor zirconium have magnetic properties. But this compound ZrZn2 has a magnetic property at temperatures below 350 degrees Kelvin.
This metal has many alloys that include brass, nickel silver, commercial bronze, tin solder, soldered aluminum. High quality zinc is used to make molds, which are used in automotive, electrical, and hardware applications.
A zinc alloy called Preztal contains 78% zinc and 22% aluminum, which is mostly used in the steel and plastics industries. This alloy is also used for molding ceramics and cement. The properties of aluminum in combination with zinc make this alloy one of the most widely used aluminum alloys and has many applications.
Zinc is also used to prevent corrosion in the plating of metals such as iron. Zinc oxide is a useful metal in the modern world. Applications of zinc oxide in industries and paints, rubber products, cosmetics and pharmaceutical industries, floor coverings, plastics, textile printing, soap making, battery storage, textiles, electrical equipment and other products.
Lithophone is a combination of zinc sulfide and barium sulfate that is used to make materials and pigments. Zinc sulfide is used to make bright, shiny X-ray and television screens and fluorescent lights. Chlorine and zinc chromate compounds are used for important compounds. Zinc is a vital element for the growth and development of animals and plants.
Zinc alone is not toxic, but when combined with oxygen in the air, it becomes a toxic substance that makes breathing difficult, and care must be taken when using it. The concentration of zinc oxide during laboratory work should not exceed 5 mg / m3 if ventilation is not required.
Specifications on |
||
| Fourth energy : 2 | Third energy : 18 | Second energy : 8 |
| Color : bluish white | Oxidation state : 2 | Atomic number : 30 |
| Fourth energy : 2 | Atomic mass : 65,409 | Melting point : 419.73 ° C |
| Boiling point : 907 ° C | Ionic radius : A 0.74 | Energy level number : 4 |
| Atomic radius : A° 1.53 | Capacity : 2 | Ionization energy : Kj / mol 9.394 |
| Periodic period : 4 | Density : 7. 13 | Electronegative : 1.65 |
| Standard Mode : Solid | Evaporation heat : Kj / mol 115.3 | |
| Group Name : 12 | Decomposition heat : Kj / mol 7.322 | |
Historical history of zinc in the world
It is the oldest piece made of beti, which was found in archeological excavations in “Dardush” in the region of Transylvania and its composition contained 87.52% zinc, 11.41% lead and 1.07% iron. . In the ruins of Kumars, which were destroyed 500 BC, a bracelet was found that was filled with zinc.
200 BC, the Romans became acquainted with brass and formed it by melting and reducing it in a kiln with copper. During this process, zinc oxide was first reduced. Zinc vapors then penetrated the copper, and as the temperature increased, the product melted and the brass was obtained with a uniform composition.
Zinc in metal and zinc oxide has been produced in India since the 12th century and was later produced and used in China in the 17th century. Around 1730 the knowledge of zinc smelting reached China from England and in 1739 the license for the distillation process was registered and during the years 1743-1740 a smelting plant with a production of 20 tons per year was established in Bristol.
In this process, a mixture of carbon and zinc oxide ore was used in a furnace with a tube at the bottom, and the evaporated zinc was liquefied in the tube and collected outside the furnace.
In the United States, zinc was first produced in 1835 in Washington. In 1880, zinc hydrometallurgy was common for the production of zinc sulfate and its use for the production of the pigment Lithapone. Until about the 1880s, little attention was paid to zinc sulfide ores, and in Europe calamine was often used to produce zinc.
However, during the 1881s, the refining process was modified and a high percentage of So2 gas was obtained, thus placing sulfur ores in the zinc production circuit.
Historical history of zinc metal in Iran
The oldest recorded mineral reports in Iran are related to Neishabour turquoise mine and Qaleh Zari copper mine, which date back to 4 to 5 thousand years BC. All the activities of the lead and zinc mines before the Second World War were carried out almost in an ancient way. In the 1930s, German experts brought a new method of mining to Iran, especially in the field of metal mines.
At this time, Nakhlak lead and zinc mines, Siah Kooh Anarak, Bibi Shahrbanu lead and several other mines were active. Lead and zinc mines flourished from 1346 to 1356. The first modern lead and zinc concentrate plant was put into operation in 1340 with the help of a French company in Lakan, Markazi province. From 1342 to 1372, the product of zinc mines in Iran after processing as a concentrate was exported abroad.
Notice: for more information please see: List of Zinc mines on Iran and list of Iran lead mines
After the imposed war, due to the existence of Angoran mine, the eyes of domestic experts turned to the acquisition of zinc metal technology. Zinc was piloted in 1992.
The production of zinc ingots in Iran Minerals Processing Company (Zanjan smelting unit) started in 1372 and from that date onwards, with the construction and operation of new units, the production of zinc ingots in the country increased. These units are mainly engaged in the production of zinc ingots using internally acquired technology.
Zinc, a natural event
In nature, the two elements lead and zinc are often associated with each other, and if you are familiar with the properties of lead metal as well as the properties of zinc metal, you know that they have common embedding stones.
About 70% of mineral lead is produced from mixed ores of lead and zinc, which typically have higher levels of zinc than lead. About 20% of lead production is related to mixed ores, which contain more lead than zinc, and the remaining 10% of lead production is related to copper ores.
Lead and zinc minerals are often found in the form of mixtures and deposits. The main deposits of these two metals are hydrothermal, epithermal, mesothermal and tele-thermal. In mesothermal deposits, the amount of zinc is usually higher.
Depending on the type of lead deposition in the cracks and joints, hydrothermal deposits form accumulation groups in which the lead in the hydrothermal solution is converted to insoluble salts due to chemical interactions and accumulates joints and cracks.
Slow and substitution in which the lead ion in the hydrothermal solution is replaced by the metal ion in the surrounding rocks, which converts, for example, calcium carbonate or lime to lead carbonate.
Lead and zinc sulfide minerals in the upper part of the deposits are affected by climate, altered and converted to oxidized minerals of this metal. It should be noted that sphalerite alters earlier than gallons. Thermal traps are usually found in calcareous layers where the veins are not heated.
In the place of these masses, recrystallization of calcite and dolomite is observed and sometimes a little gypsum and barite accompany the ore. In these deposits, sometimes lime also becomes a little siliceous. Trap-thermal deposits are usually of the substitution type.
Zinc generally occurs in the form of sulfides and in most cases from the source of hydrothermal fluids and sialic and somatic magmas. Sedimentary deposits are less important. A number of deposits formed in carbonates have the properties of marine sedimentary deposits. However, in these deposits it can be assumed that the metal is supplied through this heat transfer from the base magmas.
Exploitable zinc deposits are found in a variety of geological environments. These accumulations are divided into the following sections according to the geological conditions of origin:
- Accumulation of bulk sulfides caused by submarine eruptions in sedimentary rocks
- Stratigraphic accumulations in carbonate rocks
- Stratigraphic accumulations in sandstones
- Stacked veins
- Stacks of transformation
Zinc compounds
Zinc compounds are an important part of zinc industry products. Zinc-based chemicals, including zinc dust, account for 12 to 15 percent of the world’s zinc consumption. Zinc oxide is by far the most important chemical product based on zinc. Zinc sulfate dust and zinc chloride dust are in the next categories in terms of importance and quantity.
Other zinc compounds are of minor importance. Global consumption of zinc oxide and dust has remained stable for many years, although demand for zinc sulfate and zinc chloride is increasing.
The growth rate of zinc thiocarbonate and zinc acetate consumption is consistent with the production of abrasives (the largest consumption of these compounds). The most in-demand zinc oxide can be produced by a variety of processes.
The purity and quality of zinc oxide depends on the method of production. High purity zinc oxide is collected as a powder in sediment chambers, where particles of different sizes are separated. This substance is commonly known as white zinc.
In the direct process (or American process) the raw material used is zinc ore or zinc by-products that always contain lead. A carbonaceous material is heated with a raw material which, as a result of reduction, is reduced to zinc by steam. This substance is oxidized in air and is divided into particles of different sizes.
The main compounds of zinc are:
- Hydride (ZnH2)
- Oxidizing (ZnO)
- Zinc chloride (ZnCl2)
Also, this metal has many alloys that include brass, nickel silver, commercial bronze, solder tin, soldered aluminum. High quality zinc is used to produce molds, which are used in automotive applications and the electrical and hardware industries.
A zinc alloy called Perztal contains 78% zinc and 22% aluminum, which is mostly used in the molding steel and plastics industries. This alloy is also used for molding ceramics and cement.
Zinc is also used to prevent corrosion in the plating of metals such as iron. Zinc oxide is a useful metal in the modern world that is widely used for industries and manufacturing of paints, rubber products, cosmetics and pharmaceutical industries, floor coverings, plastics, textile printing, soap making, battery storage, textiles, electrical equipment and other products. has it. Lithophone is a combination of zinc sulfide and barium sulfate that is used to make materials and pigments.
Zinc sulfide is used to make bright and shiny screens, X-ray and TV screens, and fluorescent lights. Chlorine and zinc chromate compounds are used for important compounds. Zinc is a vital element for the growth and development of animals and plants.
The raw material for wet chemical processes is purified solutions of zinc. The carbonate or zinc hydroxide is precipitated and then filtered, washed, dried and pulverized at 800 ° C.
Various types of zinc oxide are used as intermediates in the production of other chemicals. Sedimented zinc oxide that has no dyeing properties and special grades such as fine zinc oxide used in photocopy papers. The most important application of zinc oxide is in the abrasive industry, which is used in this industry as a hardening activator and sometimes as a filter.
Zinc oxide is widely used as a dye in solution latex paints. The use of this substance in agriculture as a fertilizing additive to compensate for soil deficiency is less important. Zinc oxide is part of the formulation in the glass, glazing and ceramic industries.
This material affects the melting point, optical and elastic properties, as well as the color and luster of the glass. Zinc oxide is one of the ingredients in face powders, lipsticks and creams used in the cosmetics industry. It is also used as an additive to oils, adhesives, drying agents, opaque agents and as a catalyst in methanol synthesis.
Zinc producing countries in the world
Australia, China, Peru, Mexico and the United States have the largest reserves in the world, totaling about 152 million tons (equivalent to 74% of the world’s reserves). Of course, ores have been found in more than 50 countries. China is the largest supplier of zinc by a wide margin compared to Australia and Peru, and is also a major consumer of the metal.
Zinc processing and extraction
Zinc production methods are generally divided into two categories : pyrometallurgical and hydrometallurgical. About 85-90% of the zinc produced in the world is by hydrometallurgical method.
80% of the zinc mines are underground, 8% are open pit and the rest are a combination of open and underground. In terms of zinc metal production volume, 64% is obtained from underground (covered) mines, 12% from open pit mines and 15% from composite mines.
In the case of other metals, metal is rarely produced directly from the soil. To perform the concentration process, the mineral soil is crushed and then other metals are separated. Typically, zinc concentrate with a concentration of 55% is composed of a small amount of lead, copper and iron. The thickening process is often done on site to minimize transportation costs.
Roasting and sintering
More than 95% of the world’s zinc is produced from zinc sulfide, zinc concentrate is usually 25-30% sulfur and small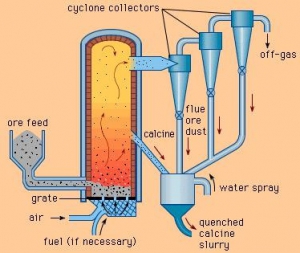 amounts of other metals such as iron, lead and silver, before zinc metal by hydrometallographic If metallurgy is extracted, the sulfur in it must be separated. This is done by washing or by sintering.
amounts of other metals such as iron, lead and silver, before zinc metal by hydrometallographic If metallurgy is extracted, the sulfur in it must be separated. This is done by washing or by sintering.
In this work, the zinc concentrate is heated to a high temperature and the zinc sulfide is converted to active zinc oxide, and immediately after that, sulfuric acid can be produced, which is an economical product.
In order to use zinc sulfide, a cleaning system is required and the direct cost of cleaning is almost equal to the costs of all parts of the zinc plant.
Pyrometallurgical method
In pyrometallurgical methods, the mineral is recovered and extracted at special temperatures by special masses. The mechanism of action of pyrometallurgical processes is to generate high heat and a suitable environment for the reduction reaction.
In pyrometallurgical methods of zinc production, the basis of the process is based on the following reactions:
The reducing agent in this process is coke metallurgy and lime is used to control playfulness. Granulation of raw materials is very important in this process. If zinc concentrate is used, it should be pelleted and if zinc oxide mineral is used, it should first be reduced to 5-10 mm granulation and used after calcination. The calcination operation is performed according to the opposite reaction.
This operation is mostly performed in rotary kilns or wagon kilns. The final smelting step is based on the reduction of zinc and lead using carbon in special furnaces designed for this purpose. This step is very costly due to the high amount of energy consumed, today this method is used only in China , India , Japan and Poland.
Hydrometallurgical method
In hydrometallurgical methods, the mineral containing the desired metal is dissolved in a suitable solvent and after separating the impurities, it is extracted by direct current through electrolysis process. Hydrometallurgical methods do not require high temperatures and metal extraction is done by electrochemical reactions.
Hydrometallurgical processes can be summarized in two ways:
- Conventional method
- Solvent Extraction Method
Due to the limited capacity of the pyrometallurgical method furnaces, increasing their number to increase the capacity is not cost effective. Therefore, the amount of zinc production in the world with this method is limited, while hydrometallurgical processes are widely used worldwide due to the possibility of economic production and high capacity, as well as the possibility of using low-grade raw materials and high impurities.
In the solvent extraction method, in which after dissolving the mineral zinc in sulfuric acid, with the help of D2EHPA organic matter, zinc sulfate with acceptable purity and the level of permissible impurities are selectively separated from the solution and by preparing a suitable solution containing Zinc is extracted by SHG (Special High Grade) with a grade of more than 99% zinc using direct current in electrowinning cells.
Investigation of hydrometallurgical process – Conventional method
The ore concentrate is transferred to the Leach unit. In the dissolution of raw materials containing zinc, it is pulped with a solution returning from the electrowinning unit (Spent) in special tanks. Fresh sulfuric acid solution is used to balance sulfate (required). After acid leaching, which is performed with the aim of dissolving the maximum amount of zinc in the mineral, the resulting slurry is pumped to the neutral leach tanks.
In these reservoirs, with the addition of lime water, the pH of the slurry increases to create suitable conditions for the deposition of some impurities such as Si, Fe, Al, Ge, As and Sb and.. The impurities are solidly separated from the solution and the solution is transferred to the treatment unit to separate other impurities. Purification of the leach solution is done in three steps.
In the first step, the concentration of chlorine ions in the solution is reduced to the permissible level by cut blue (Cu SO4). In the second stage, by increasing the temperature of the solution to 70-80 ° C and adding zinc powder and arsenic trioxide, Cu, Co and Ni impurities are separated from the solution in the form of a cake and the solution is transferred to the next stage.
In the final stage, by lowering the temperature to 50 ° C and adding zinc powder, the cadmium is sponge separated from the solution and the solution is transferred to the cooling towers.
In cooling towers, as the temperature decreases, the solubility of Ca, Mg in solution decreases, resulting in the formation of CaSO4 and MgSO4 crystals, which deposit on the floor and walls of the cooling tower and the inner part of the solution storage tank. The Ca and Mg-free solution is fed to the electrowin section. In the electrovining unit, the electrolyte passes through the cells and the Zn2 + ion is reduced and settled on the cathode and after a certain time is extracted in sheets on the SHG.
The resulting zinc foil, after being removed from the cathode in the foil unit, is washed and transferred to the smelting and casting unit. In the melting and casting unit, the sheets are charged to the induction furnace after drying.
Slagging is done with aluminum chloride and the zinc melt is cast in the form of ingots on SHG. Accuracy in operation and adjustment of work fluctuations, especially in high production capacities and recovery of maximum zinc are among the very important parameters in the economics of the zinc production process.
How it is dissolved and neutralized is the most important factor in zinc recovery. The dissolution system intended for this project can be very effective in estimating the two main objectives, namely the crude solution suitable for refining and also the high recovery of zinc from the mineral.
Due to the lack of preparation of materials, especially lead flotation operations at the beginning of the circuit and the absence of some minerals from the feed input to the zinc production process, due to the dissolution of minerals such as “mimetic” in acid, the amount of chlorine in solution increases.
For this reason, in addition to the de-magnesium phase, which reduces the concentration of magnesium, chlorine and fluorine in the circuit, a de-chlorination phase is also considered. Normally, by placing the sulfate precipitation steps on alkali (BZS) (de-magnesium depletion) and the chlorine removal step, an attempt is made to keep the concentration of chlorine, fluorine and magnesium at a concentration below the allowable level.
Leaching (dissolution)
-
Acidic leach
The pulp is sent to the acidic leach stage with a certain volume flow intensity. Most of the pulp generated, along with the returned electrolyte, is transferred to the acid leach phase tanks. Fresh sulfuric acid is added to this step to balance the sulfate needed.
The acid concentration in these reservoirs is regulated by the zinc ore. The effective parameters at this stage, such as temperature, retention time and sulfuric acid concentration, are adjusted so that the maximum extraction of the desired zinc metal is possible.
At this stage, in addition to metal, other elements such as: cadmium, cobalt, nickel, germanium, fluorine, chlorine, etc. also enter the leach solution due to dissolution. The iron that enters this stage from the Tinker bottom of the first neutralization step is precipitated by sodium sulfate as sodium jarosite and arsenic is precipitated as scorrodite (FeAsO4). The slurry from the acidic leach settles in the thickener and the thinner thinner is washed after the filter and sent to the waste treatment stage.
-
Neutralization
This operation takes place in two stages. In the first stage, the acid leach stage is neutralized by certain amounts of pulped feed in the tanks of this stage under certain conditions. After the slurry of this stage settles in the thickener, the thickener overflow is returned to the cooling towers of the solution and its discharge is returned to the acidic leach stage. In the second stage, the final neutralization and removal of gypsum is done.
The neutralizing agent in this stage is limestone and alkaline zinc sulfate slurry along with gypsum from the alkaline sulfate deposition stage (de-magnesium). Elements such as iron, aluminum, arsenic, germanium and silica remaining in the leach solution precipitate at this stage.
After concentrating the produced slurry, the Tickner overflow is first filtered and then sent to the treatment section. Part of the drain of this thickener is sent to special aluminum removal tanks and the resulting slurry is filtered and washed. Another portion of the effluent is sent to the second stage neutralization tanks and solution cooling towers.
-
Soluble purification
The crude solution (PLS) obtained from the dissolution stage contains the following impurities: copper, cobalt, nickel, cadmium and chlorine, which are harmful to the electrowinning operation. These elements are removed from the crude solution in the following three steps:
Step 1: Dechlorination
The first stage of purification (dechlorination) is the allowable concentration of chlorine in the electrolyte 200 mg/l. In order to prevent the increase of chlorine ion concentration in the electrolyte by using a separate unit to remove chlorine from zinc sulfate solution, this step is continuously controlled in terms of the amount of molar element. In this way, about 1/3 of the crude solution enters the chlorination stage, chlorination is done at pH 2.5 and temperature at about 50° C by zinc and blue cut.
Step 2: hot purification
The second stage of purification In this stage, zinc and arsenic trioxide are precipitated with copper, cobalt and nickel. To create favorable conditions for complete deposition of Cu3As, CoAs and NiAs, certain amounts of copper sulfate are used.
The temperature of this stage is considered between 70-80 degrees Celsius at PH = 4.5. After filtration, copper, cobalt and nickel deposits are washed with water and stored in the warehouse for further processing or sale.
Step 3: Cold Purification
At this stage, by adding zinc powder at a temperature of about 50 ° C and adjusting the pH by the electrolyte returning from the electrovining stage (PH = 4), cadmium is precipitated into a sponge. It is sent to the electrowinning stage.
Strontium or barium carbonate is added to the cycle to precipitate lead to the electrolyte. Lamination of aluminum cathodes is done mechanically after a certain period of time.
Melting and casting unit
The zinc sheets produced in the electrowinning unit are first stored on a storage platform to cool naturally by air. The dried sheets are weighed by a weighbridge and fed to the induction furnace by a forklift. In this part, ammonium chloride is added to reduce the production of metal slag to prevent the loss of zinc metal.
The metal on the melt is transferred to the ingot casting machine by a special pump through a gutter. The ingots are stacked, weighed and packed and transported to the warehouse by forklift.
The main difference between the solvent extraction method and the conventional method is in the purification section. In the dissolution extraction method, the zinc dissolved in the leach solution is selectively separated from the other impurities, while in the conventional method, the impurities are separated from the leach solution and the pure zinc sulfate solution is extracted. Therefore, the preparation (crushing, smoothing and milling), leaching, electrovining, smelting and casting, etc. are similar in both methods.
Comparison of pyrometallurgical methods with hydrometallurgical methods of zinc production:
- Pyrometallurgical methods are not cost-effective at high production capacities, but hydrometallurgical methods at high production capacities are also quite economical.
- Hydrometallurgical methods are also used for low grade ores but in pyrometallurgical methods only high grade ores can be used.
- Pyrometallurgical methods are sensitive to changes in the chemical composition of raw materials so that if the amount of some impurities is too high, production stops or the work efficiency of the process is severely reduced.
- Energy consumption in pyrometallurgical processes is usually higher.
- Due to the lack of effluent in pyrometallurgical processes and the ability to reuse the resulting waste in these methods, environmental issues in pyrometallurgical methods are less.
Comparison of conventional method with solvent extraction method or SX (Solvent Extraction)
- The SX method has a great deal of flexibility in changing the chemical composition of zinc raw materials, so that the use of zinc raw materials containing large impurities does not affect system performance. But in the conventional method, the process changes somewhat based on the amount of impurities.
- The high quality of the electrolyte in the SX method leads to the use of lower electrical voltages in the cells, which in turn results in less electrical energy consumption.
- The level of fluorine and chlorides in the section space (EW) in the SX method is much lower than the conventional method, so the EW section environment is free of chlorine gases and thus prevents corrosion of buildings, equipment and tanks.
- The cost of building a factory with the SX method is higher than the conventional method.
- The SX method has been more successful than the conventional method in obtaining SHG quality.
Thus, although the SX method has significant advantages overall compared to the conventional method, the initial investment required is higher than the conventional method. Due to the existence of several active units and the technical knowledge of the conventional method in the country, this method (taking into account all aspects), is usually the first choice of zinc production units in Iran.
Table 9: The amount of raw materials required to produce one ton of zinc ingots
name of the material |
Kg per ton of ingot production |
| Zinc concentrate | 4200-4300 |
| sulfuric acid | 800-1000 |
| Hydrated iron | 250-260 |
| Iron sulfate | 40-50 |
| Manganese dioxide | 30-35 |
| Aluminum sulfate | 25-30 |
| Copper sulfate | 7-8 |
| Lead for the Andes | 2.5-3 |
| Silver for cathode | 0.025-0.3 |
| Manganese sulfate | 1.5-2 |
| Strontium carbonate | 0.15-0.2 |
| salt | 150-160 |
| Potassium permanganate | 0.08-0.1 |
| Filter cloth press | 0.05-0.055m2 |
Instructional video of zinc ingot production process in the factory
Applications of zinc metal
About 12 million tons of zinc are produced annually in the world. More than half of this amount is used in the galvanizing industry. About 14% is used in zinc-based alloys, which are mostly used in the die casting industry, and 10% is used in the production of brass and bronze.
Significant amounts are consumed together in rolled zinc, which includes roofing, gutter, down pipe. The rest is used in compounds such as zinc oxide and zinc sulfate, with first-hand suppliers covering a wide range of zinc applications.
The main applications of zinc metal are :
- construction industry
- Transportation
- Food consumption
- Electrical and electronic appliances
There are other coating methods that are used. Like zinc-enriched paints, electrostatic zinc, mechanical methods, these methods are very different from galvanizing and may not be suitable for some environments.
Currently, 75% of the zinc used in the world is supplied from mines and mineral soils and 25% from recycled zinc. The amount of recycling is increasing every year. The amount of zinc recycling depends entirely on the amount of zinc products collected after consumption. More than 90% of these products are collected after consumption. Zinc can be recycled at all stages of production and consumption.
For example: rom zinc waste in the production of galvanized sheet to waste from production and installation processes and also at the end of service life.
Zinc-coated steel and other zinc-containing products enter the recycling cycle due to their long service life after a long period of production. The durability of products containing zinc varies from 5 to 50 years in automobiles and home appliances to more than 100 years in galvanized sheets. Zinc-coated street lights can last for more than 40 years.
Over the past century has increased the lifespan of steel. Zinc coating is the most economical way to increase the protection of steel against corrosion, which annually destroys about 4% of the gross domestic product of non-industrial countries.
Galvanized steel has properties that are not provided by any other material. In addition to the mentioned properties, the electrochemical property of zinc has led to its use in the production of batteries. Among the most important and newest applications of zinc metal and its modified alloys, its use in the manufacture of electronic devices such as mobile phones, tablets and other items mentioned.
Expenses of zinc metal
Zinc in cement making, dentistry, match making, flooring, pottery, rubber accessories, car making, kitchen appliances, steel coating (galvanizing), preparation of bronze and brass alloys, soldering, toothpaste cans, typewriter metal glue , German silver and..
Zinc oxide and sulfur are used as white pigments in paint and plastic production, zinc sulfate is used in dyeing and adhesive making, and zinc chloride is used in soldering and wood decay prevention.
Zinc anodes are used to prevent corrosion of ship hulls, drilling rigs and underwater pipelines. If zinc is used to make zinc or brass plates, the size of its aluminum should not exceed 0.005%. The amount of tin in the high grade zinc grade should not exceed 0.001%. The amount of aluminum in the PW type should not exceed 0.05%.
Intense electrochemical activity prevents cathodic corrosion in iron and steel products. Zinc alloying with copper and forming a brass alloy that has properties such as usability at low temperatures, corrosion protection and beautiful polishing.
Two other grades of zinc have been accepted for galvanizing purposes. One is called the Continuous Galvanizing Grade, which has up to 35% lead and some aluminum, and the other is called the Controlled Lead Grade, which has less than 18% lead and is far from aluminum.
Among the important cases of zinc use in various industries, the following can be mentioned:
- Zinc is used as part of battery tanks in foundry molds and in the automotive industry.
- Zinc oxide is used as a white pigment in blue and various paintings.
- Zinc chloride is used as an anti-odor (body spray) and as a wood preservative.
- Zinc sulfide is used in light pigments to make clock hands and other objects that glow in the dark.
- Methyl zinc [Zn (CH3) 2] is used in the synthesis of organic matter.
- Solutions (lotions) made from calamine are a mixture of carbonates and silicates on Silicate and Zn-hydroxy-Carbo that are used to treat acne.
Construction industry
Zinc is also used in civil works. Although zinc has a high resistance. It was not used as a building material for a long time due to its very low creep resistance. Zn-Ti-Cu low zinc alloys with excellent malleability and high creep resistance have been developed over 40 years.
These materials can be rolled to produce zinc plates or sheets. These panels can be used in the production of roof drainage equipment or in covering buildings.
Alloys
Zinc recrystallizes at temperatures above room temperature and has a low creep resistance. Therefore, this element is only suitable as a building material when alloying.
Alloy elements, mostly Al, Cu, Mg, lead to grain shrinkage, mixed crystals or sediment hardening, and thus the mechanical properties of the metal are developed. Zinc is used in the production of brass, silver and nickel alloys. Zinc is also used in foundry and automotive industries.
Galvanizing steel
Zinc metal is used in galvanizing steel products and prevents corrosion of steel. Most of these products are used in galvanizing steel, roofing sheets, storage tanks, fences.
Galvanized sheets are also used as air ducts in air conditioning systems and ventilators and heating systems.
Zinc coating protects against corrosion by air through the following mechanism:
- The effect of carbon dioxide and air humidity on the formation of a protective coating based on zinc carbonate, which leads to a reduction in corrosion rate.
- Due to the electrochemical properties of cathodic protection, zinc actively prevents corrosion in small damaged areas, damaged parts and cut edges of plates.
- Optimal use of the world’s raw material resources requires that we protect metals from corrosion. As the atmosphere becomes more corrosive, corrosion protection standards must increase, especially in steel production. In industrialized countries, approximately 50% zinc is used to prevent corrosion.
Commercial zinc (99.5% – 98.5%) is used for hot-deep galvanizing in production and distribution equipment and high purity zinc (99.95%) is used for continuous galvanizing of springs and steel wires.
The metallurgical process used to produce zinc of lower purity is the use of lead as the main impurity. The presence of 1% lead reduces surface stress by 40% compared to purer products. When immersed in a zinc bath that lasts only a few minutes, the metal heats up to 4,500 degrees Celsius.
The diffusion process leads to the formation of a layer of iron-zinc alloy on the steel surface, which increases the adhesion of the zinc coating. In addition to hot-deep processes, other galvanizing processes have been developed.
On electrolytic coating, an alternating current leads to the deposition of a shiny coating of 25-5 cm of acidic, cyanide or cyanide-free cyanide electrolytes on the surface. Of course, this coating only protects the metal against moderate surface corrosion.
Due to the high ratio of zinc in the oxidation and reduction potential, this metal is used to protect iron and steel against corrosion. Zinc metal produced from ore is called primary zinc or first hand.
The zinc metal from the tailings residues and scraps are called secondary, redistilled or re-melted depending on the type of recovery process. Primary zinc is classified into two categories: electrolytic zinc and distilled zinc, according to the reduction method used.
Electronic
Zinc metal is used in electrical wires, telecommunications and bolts. Zinc oxide is used as a sheet in the body of dry batteries, the roof covering of photographic engraving plates, to protect the hull of pipelines and drilling facilities at sea, in addition to plating bolts and nuts and small metal parts, deposition of metals Rare in a solution, lead desalination is used by the Parkes process, separation of impurities such as copper, cadmium and nickel from a solution (before electrolysis) from zinc dust is used.
Zinc Ferrite is used in electronic components in transformers (coils), amplifiers, motors, tuners, as well as in electronic components in radio, television, and computers.
Painting
Zinc oxide is used in the dyeing industry due to its opacity and high refractive index (High Refractive Index), which increases the color fastness.
Rubber Manufacturing
Zinc oxide is also used as the main activator and accelerator in the sulfur-tightening industry. Lito Pone, a barium-zinc sulfate pigment, is used in the paint, rubber and printing industries.
Pharmacology
Smith Zonite is known by the old name of calamine in medicine and the substance used in the pharmaceutical industry is ZnO from Smith Zonite, the purity of which must be more than 98%. It is insoluble in water as a pale pink powder but dissolves well in mineral acids.
Calamine has an anti-itching effect and it is introduced for external use (skin) and in the form of powder, sunscreens (lotions) with the following brand names:
- Aqueous Calamine Cream
- Oily Calamine Lotion
- Calamine Ointment
- Calamine Lotion
Calamine is also used in compounds of a drug called calamux, which is made in the United States. Calamine-based medicines are used for dry skin, mild burns, eczema, abrasions, and more.
Zinc Oxide is used in the processing of other zinc compounds used in the manufacture of products such as inks, hair dyes, oil additives, antifungal drugs, polishes and linoleum.
Casting under pressure with zinc
Zinc die-cast is one of the main applications of zinc by ZnAl4Cu alloys and other related compounds.
Some of the advantages of zinc in pressure casting are:
- Production speed can be very wide (1000-60 pieces) depending on the size and complexity of the parts.
- Low viscosity and low melting temperature of alloys guarantee high quality product.
- Low and controllable shrinkage of zinc alloys increases dimensional accuracy.
- Glossy or matte surfaces can be achieved with a variety of coatings.
- Due to the wide range of production, costs are low. The products are highly durable due to their good mechanical and physical properties.
- The energy required for these processes is low. About 50% of pressurized cast parts are used in the automotive industry.
- Pressurized zinc is also widely used in the manufacture of doors and windows, furniture, locker cabinets, switches, faucets and bathroom fittings.
Automotive industry
Zinc is also widely used in the automotive industry, including in the construction of networks, lock handles, mechanical and electrical components, body, and electrical connections.
printing industry
Zinc oxide is also used in the printing industry (photocopying). Zinc compounds are also used in the manufacture of anti-corrosion paints for chemical catalysts, welding melts, cathode ray tubes, radar plates, and additives in lubricating oils and greases.
Fire-fighting equipment and wood storage
Zinc sulfate and zinc chloride are two other important compounds present in fire protection equipment for the maintenance of wood and molten materials.
Agricultural industries
It is used in making fertilizer as an additive.
Metal on the secondary
The recovered zinc metal is increasingly entering the raw material supply chain. These by-products are very economical in terms of energy consumption and cause less pollution from the metal production process from the concentrate. The mentioned advantages have caused metal smelting centers to be interested in zinc recycling.
Although the main reason is to recover its economic benefits, environmental issues are also of particular importance. Manufacturers in developed countries now have to consider the fate of a product after its useful life, even before designing and building it.
Restrictions on landfills and concerns about the disposal of hazardous materials add to these issues. The American Zinc Trading Company, a recovery company, estimates that about 10 million tons of zinc are consumed annually in the world, 70% from ore and 30% from waste recovery. More than half of the lead and zinc used today are recycled.
Zinc consumption in Iran and the world
The outlook for zinc for global lead and zinc is that global zinc consumption will grow by 4.8% to 10.34 million tonnes in the next two years and by 4.3% to 10.78 million tonnes over the next two years. Increase the period 2018-2014.
The main reason for this is the increase in consumption in the galvanizing industry in China and investment in infrastructure projects such as the construction of new roads, railways and transmission facilities, the rapid development of housing and the automotive industry.
Due to easy access to raw materials (zinc ingots) for consumer industries in Iran, especially in the field of galvanizing and oxide production, its consumption has increased significantly so that according to information obtained, domestic consumption has reached about 70,000 tons this year.
The highest apparent consumption during these years (2014-2014) is 13 million tons. The apparent consumption of lead and zinc in the world during 2010 and 2011 had a relatively constant amount, and considering the constant global production in these years, it can be said that a relative balance between supply and demand has been established.
What factors affect price forecasts?
In general, despite the expectation that zinc prices will rise, prices cannot be expected to soar. China has great potential to be activated if the price of the metal jumps a lot. Although the data show an increase in the price of zinc, the following factors affect these predictions, some of which can lead to higher prices and others can lead to lower prices.
Some of these causes are as follows:
- Delay in new mine development
- Production on smelters in China will not increase
- Continue per capita consumption of China as well as Japan, South Korea and Taiwan
- Increasing the acceleration of zinc consumption as a feedstock in agricultural fertilizers
- A sharp increase in the use of galvanized sheets in the automotive industry
- Replacement of galvanized sheets with other materials in the automotive industry
- Uncontrollable liberalization of inventory
- A sharp increase in the supply of zinc by Chinese mines
- Irregular balance in the Chinese economy
- Revival of production on smelters in China
Raw materials needed to produce zinc ingots
The main raw material for the production of zinc ingots is zinc ore in the form of sulfate or carbonate, but other by-products for the processing of zinc sulfide ore are used, which are mentioned below.
- Burning profit
- Sodium hydroxide
- lime water
- sulfuric acid
- Silica and…
Machines and equipment for processing zinc sulfide ore to produce zinc ingots
- Flotation line
- Vibrating feeder
- Jaw crushers
- Hydraulic shredders
- Vibrator plate
- Save between
- Pendulum feeders
- Ball Mill
- Spiral calcifier
- Stirring tanks
- Flotation device
- Magnetic separators
- Tube mixers
- Dryers and conveyors
- Spiral washers
- Electrolysis equipment
- Casting and melting equipment
- Metal smelting furnace
- Storage tanks for chemicals such as sulfuric acid and a variety of solutions
- Cooling tower and purification reactor
- Types of pumps
- 25-22 kg molds for producing ingots and…
- Equipment for oxygen production line with nitrogen and argon
Conclusion
Zinc is still abundant in the earth’s crust, and zinc reserves are growing, exploration continues, and mines and smelter projects are still coming into production. But the point is that with the current price of zinc, the efficiency of mining projects and smelters is low.
Therefore, when the price of zinc rises, it will be easier to finance these investments due to better returns. But they can take years to complete.
In the meantime, there is a consensus that the supply deficit will continue until new mines and smelters enter production. This deficit requires users to stockpile.
On the other hand, as these stocks decline, the price of zinc is more likely to rise. if you want buy zinc from Iran please contact us.
Notice: Translated by google
